- Introduction to Osmosis
- Osmosis Definition
- Explanation of Osmosis
- Types of Osmosis
- Osmotic Pressure
- Significance of Osmosis
- Introduction to Osmosis
Osmosis, the silent superstar of biology, holds the key to some of life’s most essential processes. In this journey, we’ll dive deep into this fascinating phenomenon and explore its definition, mechanics, types, the pressure it exerts, and its incredible significance in our world.
- Osmosis Definition
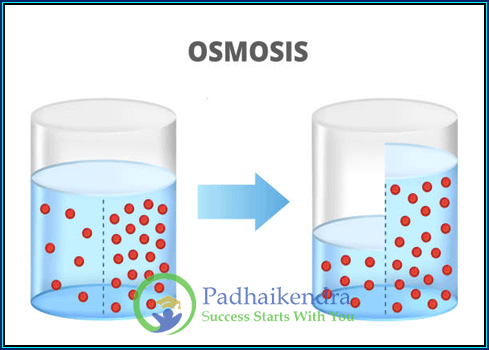
Let’s start with the basics. What exactly is osmosis? Picture a scenario where you have two containers separated by a semi-permeable membrane. Osmosis is the magical movement of water molecules through this membrane, from an area of lower solute concentration to an area of higher solute concentration. It’s like water’s secret way of sneaking through barriers.
- Explanation of Osmosis
To understand osmosis, imagine you’re at a crowded party. If you’re at the emptier side of the room (low solute concentration), you naturally want to move to the more happening side (high solute concentration). Water molecules, being the party-goers they are, do the same. They move through the semi-permeable membrane to join the solute-rich side, creating balance.
- Types of Osmosis
Osmosis comes in different flavors. We have hypertonic, hypotonic, and isotonic solutions. Hypertonic is like being at a dance party where you’re the only one dancing (more solutes outside the cell). Hypotonic is the crazy dance-off with a bunch of wild dancers (more solutes inside the cell). Isotonic is that perfect dance party where everyone dances in harmony (equal solutes inside and outside the cell).
- Osmotic Pressure
Now, let’s talk pressure. Osmosis isn’t just a random dance; it’s a high-energy dance party! Osmotic pressure is the force exerted by water as it pushes to equalize solute concentrations. It’s like the dance floor getting crowded because everyone wants to party together.
- Significance of Osmosis
Osmosis is the unsung hero of life. It’s the reason plants stand tall and animals stay hydrated. In our bodies, it’s what keeps our cells happy and balanced. Imagine your cells as tiny osmotic dancers, grooving to the rhythm of life.
In conclusion, osmosis might sound like a complicated dance move, but it’s a fundamental process that keeps the show going in the world of biology. Understanding its definition, mechanics, types, pressure, and significance can open doors to unraveling the mysteries of life itself. So, keep dancing, water molecules!
- Mechanisms and Concepts of Osmosis
- Solutions in Osmosis
- Understanding Osmotic Effects
- Examples of Osmosis in Biological Systems
- Mechanisms and Concepts of Osmosis
Welcome back to our exploration of osmosis, where we’ll dive deeper into the mechanisms and concepts that make this phenomenon tick. In this chapter, we’ll unravel the role of solutions, understand the effects of osmosis, and witness real-life examples of this intricate process in biological systems.
- Solutions in Osmosis
At the heart of osmosis lies the concept of solutions. Think of solutions as mixtures. They can be as simple as a glass of sugar water or as complex as the fluid inside your cells. In osmosis, water dances with these solutions. It’s the solutes (like sugar or salt) in these solutions that dictate the dance moves. More solutes mean a wilder dance, while fewer solutes lead to a calmer groove.
- Understanding Osmotic Effects
Now, let’s talk about the effects of osmosis. Imagine you have a raisin and a grape sitting next to each other. The grape is plump and juicy, while the raisin is all shriveled up. What’s the secret? Osmosis! The grape has a higher concentration of water inside, so water moves in and makes it swell. The raisin, with less water, loses water and shrinks. This is osmotic effect in action, where water flows to balance things out.
- Examples of Osmosis in Biological Systems
But osmosis isn’t just a theoretical concept; it’s a star player in the biological world. Let’s peek at some real-life examples:
Cell Membrane Magic: The cell membrane is like a bouncer at a party. It lets the right molecules in and keeps the wrong ones out. Osmosis helps in this selection process, ensuring that only the coolest molecules get to enter the cell.
Plants’ Water Uptake: Ever wondered how plants stay hydrated? Osmosis is the answer. Water moves into plant roots through osmosis, helping them stand tall and proud.
Kidney’s Filtration: Your kidneys are master osmotic regulators. They filter your blood, ensuring the right amount of water and solutes stay in your body while the rest are sent packing as urine.
Red Blood Cells: These little blood heroes rely on osmosis to stay in shape. In a hypotonic solution, they swell up like balloons, and in a hypertonic solution, they shrink like deflated balloons.
In the biological world, osmosis is the conductor of the orchestra, ensuring that the right notes are played at the right time. It’s a process that keeps our cells happy, our plants thriving, and our bodies functioning.
So, as you dive deeper into the world of osmosis, remember that it’s not just a scientific concept; it’s a force that orchestrates the rhythm of life itself.
III. Applications and Implications
- Osmosis in Cellular Biology
- Cell Membrane Structure
- Selective Permeability
- Osmosis in Plant Cells
- Osmosis in Animal Cells
- Osmosis in Physiology
- Osmosis in Kidney Function
- Osmosis in Red Blood Cells
- Osmotic Disorders and Health
- Hypertonic, Hypotonic, and Isotonic Solutions
- Dehydration and Osmotic Balance
- Edema and Osmosis
- Laboratory Methods for Studying Osmosis
- Osmosis Experiments
- Osmotic Potential Measurement
- Use of Semi-permeable Membranes
III. Applications and Implications
Now that we’ve got a grasp of the dance moves in osmosis, it’s time to explore its applications and implications in the real world, especially in the realms of cellular biology, physiology, health, and scientific research.
- Osmosis in Cellular Biology
Cell Membrane Structure: The cell membrane, akin to a bouncer, controls who gets in and who stays out of the cell. Osmosis is the bouncer’s secret language, letting it communicate with the molecules trying to gain entry. Understanding osmosis helps us understand this vital cellular security.
Selective Permeability: Cells aren’t just letting anyone crash the party inside. Osmosis ensures that only the right molecules get a VIP pass, keeping the cellular environment just right.
Osmosis in Plant Cells: Plants aren’t immune to the charms of osmosis either. It’s what helps them absorb water through their roots, making them stand tall and proud, even on the hottest of days.
Osmosis in Animal Cells: Animal cells are osmotic wizards too. When they find themselves in different types of solutions, like the hypotonic pool of your bloodstream or the hypertonic ocean of your salty tears, osmosis ensures they stay in shape.
- Osmosis in Physiology
Osmosis in Kidney Function: Your kidneys are like the body’s water managers. They use osmosis to filter your blood, keeping the right stuff in and kicking out the waste. It’s a delicate dance that keeps you healthy.
Osmosis in Red Blood Cells: Red blood cells are the couriers of oxygen, and they depend on osmosis to maintain their ideal shape and size. They swell or shrink depending on the osmotic conditions, making sure they can navigate the tightest capillaries.
- Osmotic Disorders and Health
Hypertonic, Hypotonic, and Isotonic Solutions: These terms aren’t just fancy words; they describe the balance of osmosis in your body. Understanding them helps us comprehend why drinking seawater isn’t a good idea.
Dehydration and Osmotic Balance: Ever felt parched on a hot day? That’s your body’s osmotic sensors signaling a water shortage. Maintaining osmotic balance is crucial for your well-being.
Edema and Osmosis: Edema, or swelling, occurs when osmosis goes haywire. It’s like inviting too many people to a party – things get crowded, and it’s not fun. Osmosis plays a role in managing this condition.
- Laboratory Methods for Studying Osmosis
Osmosis Experiments: In the lab, scientists use osmosis to understand how different solutions affect living things. These experiments reveal essential insights into biology, health, and even food preservation.
Osmotic Potential Measurement: It’s not always about watching the dance; sometimes, it’s about measuring the rhythm. Osmotic potential measurements help scientists quantify osmosis, leading to groundbreaking discoveries.
Use of Semi-permeable Membranes: Imagine membranes as dance floors; some are open to everyone, and others are exclusive. Scientists employ semi-permeable membranes to understand the selective nature of osmosis better.
In conclusion, osmosis isn’t confined to textbooks and classrooms; it’s a dynamic force that shapes the biology of life itself. Its role in cellular biology, physiology, health, and scientific exploration highlights its significance in understanding our bodies, the natural world, and the mysteries of science. As we move forward, remember, the dance of osmosis is happening all around us, in every cell and in every drop of water.
- Industrial and Technological Applications
- Reverse Osmosis
- Osmosis in Food Preservation
- Osmosis in Pharmaceutical Processes
- Osmosis in Emerging Technologies
- Osmosis in Nanotechnology
- Osmosis in Drug Delivery
- Industrial and Technological Applications
We’ve journeyed through the fascinating world of osmosis in biology, physiology, and health. But did you know that osmosis isn’t confined to the realms of living organisms? It’s a powerhouse phenomenon with a slew of practical applications in industry and technology. Let’s unravel these applications, from the magic of reverse osmosis to its role in emerging technologies.
- Reverse Osmosis
Imagine a world where you could purify water without boiling it, using chemicals, or lugging heavy filters. Well, welcome to the realm of reverse osmosis (RO), where osmosis works in reverse. It’s like a bouncer at a nightclub who only lets the cool people in, except in RO, we’re filtering out contaminants.
Reverse osmosis is the go-to method for turning seawater into drinking water, removing impurities, and making it safe for sipping. It’s the technology behind those sleek home water purifiers and desalination plants that quench the thirst of coastal communities. RO systems are like molecular bouncers, letting only water molecules through and keeping pollutants on the other side.
- Osmosis in Food Preservation
When it comes to food, osmosis isn’t just about water dancing around. It plays a pivotal role in food preservation, making sure your pickles stay crunchy and your fruits remain juicy.
Take pickles, for instance. They’re soaked in a solution containing high salt or vinegar concentration. Osmosis steps in, drawing water out of the pickle cells and into the solution. This makes the pickle less hospitable for spoilage-causing bacteria. The same principle applies to jam making, where osmosis helps create that perfect jelly consistency.
- Osmosis in Pharmaceutical Processes
In the world of pharmaceuticals, precision is paramount. Osmosis lends a helping hand in drug formulation and quality control.
Picture a pill that dissolves slowly in your stomach to deliver medicine over time. Osmosis makes it possible. Drug manufacturers can create pills with semi-permeable membranes that allow water to seep in gradually, controlling the drug’s release. It’s like a timed-release party for medication inside your body.
- Osmosis in Emerging Technologies
Now, let’s dive into the future. Osmosis is making waves in emerging technologies that could redefine how we live and interact with the world.
Osmosis in Nanotechnology: Nanotechnology is all about building tiny things with incredible precision. Osmosis is helping scientists manipulate molecules at the nanoscale. It’s like orchestrating a molecular dance, where osmosis dictates which molecules go where, allowing for precise assembly of nanosized structures.
Osmosis in Drug Delivery: Imagine a world where medicine is delivered with pinpoint accuracy, targeting only the affected cells. Osmosis is at the forefront of this revolution, making it possible to design drug delivery systems that respond to the body’s needs. It’s like a smart drug that knows exactly where to go.
In conclusion, osmosis isn’t just a biological dance; it’s a universal rhythm that transcends living organisms. Its applications in reverse osmosis, food preservation, pharmaceuticals, and emerging technologies showcase its versatility and potential to shape our future. From quenching our thirst to revolutionizing drug delivery, osmosis is a phenomenon that’s making waves far beyond the biology textbooks. So, keep an eye out for its subtle moves in the world around you; it’s more influential than you might think.