In the context of thermodynamics, there exists a fascinating concept known as latent heat. It plays a significant role in understanding phase transitions, such as the conversion of ice to water or water to vapor. In this article, we will explore the world of latent heat, its definition, its different types, and its importance in everyday life.
Latent Heat Definition
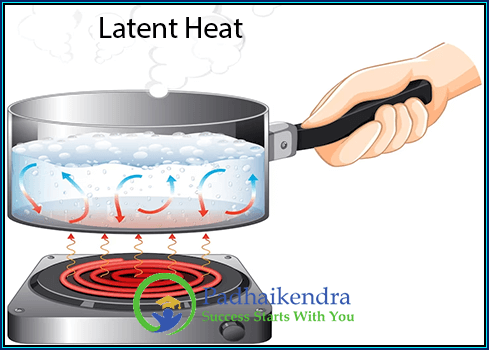
Latent heat refers to the heat energy absorbed or released by a substance during a phase change without a change in temperature. It is the energy involved in the transition of a substance from one state to another, such as solid to liquid or liquid to gas, while the temperature remains constant.
Types of Latent Heat
Latent Heat of Fusion
 The latent heat of vaporization is linked to the transition from a liquid to a gas state. The latent heat of fusion is the amount of heat energy required to change a substance from its solid state to its liquid state at its melting point. During this change of state, the substance absorbs heat energy without a corresponding increase in temperature. This is because the added energy is used to break the bonds between the molecules of the solid, allowing them to move freely in the liquid state. The latent heat of fusion is a characteristic property of each substance, and it is usually expressed in joules per gram (J/g).
The latent heat of vaporization is linked to the transition from a liquid to a gas state. The latent heat of fusion is the amount of heat energy required to change a substance from its solid state to its liquid state at its melting point. During this change of state, the substance absorbs heat energy without a corresponding increase in temperature. This is because the added energy is used to break the bonds between the molecules of the solid, allowing them to move freely in the liquid state. The latent heat of fusion is a characteristic property of each substance, and it is usually expressed in joules per gram (J/g).
When a solid substance, like ice, is heated, it absorbs energy from its surroundings to break the intermolecular bonds and transform into a liquid, such as water. Conversely, when a liquid substance freezes, it releases the same amount of energy into its surroundings.
Latent Heat of Vaporization
 The latent heat of vaporization is linked to the transition from a liquid to a gas state. Latent heat of vaporization is the amount of heat energy required to change a substance from its liquid state to its gaseous state at its boiling point. During this change of state, the substance absorbs heat energy without a corresponding increase in temperature. This is because the added energy is used to break the intermolecular bonds between the molecules of the liquid, allowing them to move freely in the gas state. The latent heat of vaporization is also a characteristic property of each substance, and it is usually expressed in joules per gram (J/g).
The latent heat of vaporization is linked to the transition from a liquid to a gas state. Latent heat of vaporization is the amount of heat energy required to change a substance from its liquid state to its gaseous state at its boiling point. During this change of state, the substance absorbs heat energy without a corresponding increase in temperature. This is because the added energy is used to break the intermolecular bonds between the molecules of the liquid, allowing them to move freely in the gas state. The latent heat of vaporization is also a characteristic property of each substance, and it is usually expressed in joules per gram (J/g).
When a liquid, like water, is heated, it absorbs energy to overcome the intermolecular forces and gets converted to vapor, such as steam. Conversely, when vapor condenses to form a liquid, it releases the same amount of energy.
Importance of Latent Heat
Latent heat plays a crucial role in many aspects of our daily lives. Here are a few examples:
Heating and Cooling: Latent heat is responsible for the cooling effect when we sweat. As perspiration evaporates from our skin, it absorbs heat from our bodies, providing a cooling sensation.
Cooking and Food Preservation: Latent heat is utilized in cooking and food preservation processes. When water boils, it absorbs heat, allowing food to cook. Similarly, freezing food involves the release of latent heat, helping to preserve it by removing heat energy.
Climate and Weather:
 Latent heat is central to atmospheric phenomena. When water evaporates from oceans, lakes, and rivers, it absorbs energy from the surroundings. As the moist air rises and cools, the water vapor condenses, releasing the latent heat and fueling the formation of clouds, rain, and other forms of precipitation.
Latent heat is central to atmospheric phenomena. When water evaporates from oceans, lakes, and rivers, it absorbs energy from the surroundings. As the moist air rises and cools, the water vapor condenses, releasing the latent heat and fueling the formation of clouds, rain, and other forms of precipitation.
Heating Systems: Latent heat is harnessed in heating systems, such as steam radiators. Steam carries a significant amount of latent heat energy, which is released as it condenses on colder surfaces, providing warmth.
Latent heat is a captivating concept that explains the energy exchange during phase transitions. It influences everyday phenomena, from the cooling effect of sweating to the formation of rain in the atmosphere. By understanding latent heat, we gain insights into how energy is absorbed or released when substances change states. So, the next time you witness ice melting or water boiling, remember the hidden energy behind these transformations, as latent heat works its magic.