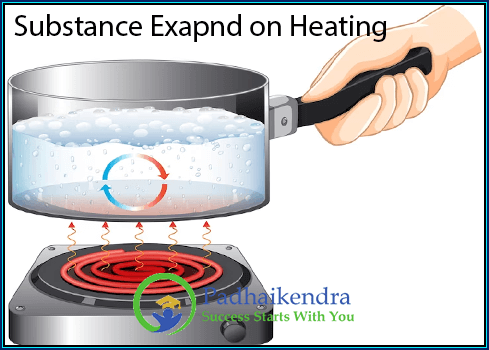
Why Do Substances Expand on Heating?
At a basic level, most substances expand when they are heated due to the increase in the energy of their particles, be they atoms, molecules, or ions. Substances expand when heated due to an increase in the kinetic energy of their constituent particles (atoms, molecules, or ions). As heat is added to a substance, the kinetic energy of its particles increases, causing them to move more rapidly and randomly. This increase in movement results in an increase in the average distance between the particles, leading to an increase in the size and volume of the substance.
Factual Explanation for “Why do substances expand on heating?”:
Molecular motion: All substances are made up of tiny particles that are always in motion. When you heat a substance, you’re giving its particles more energy. This makes them move faster and push against each other, causing the substance to expand.
Bond expansion: In substances, particles are held together by bonds. When heated, these bonds can vibrate more vigorously, leading to an increase in the average distance between particles, causing expansion.
Density decrease: As substances expand upon heating, their density usually decreases because the same amount of matter occupies a larger volume.
Amount of Expansion – The exact amount of expansion depends on the properties of the substance, such as its coefficient of thermal expansion, which measures how much the substance expands or contracts for a given temperature change. The coefficient of thermal expansion varies from one material to another and depends on factors such as the atomic or molecular structure, bonding, and density.
Examples:
Railway tracks: In India, railway tracks have small gaps between them. During summer, when temperatures soar, the metal tracks expand. These gaps prevent the tracks from buckling or warping due to this expansion.
Bimetallic strip in thermometers: Many thermometers use a bimetallic strip made of two different metals. As they’re heated, the metals expand at different rates, causing the strip to bend. This principle is used to measure temperature changes.
Overhead electrical wires: In Indian summers, overhead electrical wires can sag due to the expansion of the metal on heating. This is why they’re often installed with a bit of slack.
Importance
The expansion or contraction of a substance due to changes in temperature can have important practical applications in fields such as engineering, physics, and materials science. For example, it is crucial to take into account the expansion or contraction of materials when designing structures that are exposed to changes in temperature, such as bridges, buildings, and pipelines.
By understanding the basic principle of particle motion and the effects of heat on substances, it becomes clearer why things like railway tracks in India need gaps and why electrical wires sag in the summer heat. It’s all due to the expansion of substances upon heating!
Related Questions:
Do all substances expand at the same rate?
No, different substances have different coefficients of thermal expansion, which means they expand at different rates when subjected to heat.
Are there substances that contract when heated?
This is rare, but there are materials known as “negative thermal expansion materials” that contract when heated over certain temperature ranges.
What happens when substances are cooled?
Generally, when substances are cooled, their particles lose energy, move closer together, and the substance contracts.
FAQs
Que. Why do substances expand when heated?
Sol. Substances expand when heated because the increase in temperature causes the molecules or atoms within the substance to gain energy. This increase in energy leads to an increase in the average kinetic energy of the particles, causing them to move more vigorously and occupy a larger space, thus resulting in expansion.
Que. What causes the particles in a substance to move faster when heated?
Sol. Heating a substance increases the thermal energy of its particles. This added energy increases the amplitude of their vibrations or movements, making them move faster. The increased thermal energy overcomes the attractive forces between particles, allowing them to move more freely and occupy a larger volume.
Que. Does every substance expand when heated?
Sol. No, not every substance expands when heated. The expansion of a substance depends on its coefficient of thermal expansion. Some materials have a higher coefficient of expansion, causing them to expand more significantly with temperature changes, while others have a lower coefficient of expansion and expand to a lesser extent.
Que. Does the expansion of a substance occur uniformly?
Sol. The expansion of a substance may or may not occur uniformly. In some cases, substances expand uniformly, where all dimensions (length, width, and height) increase proportionally. However, in other cases, substances may exhibit different expansion rates in different directions, leading to non-uniform expansion.
Que. Can the expansion of a substance be reversible?
Sol. Yes, the expansion of a substance can be reversible. When the substance is heated and expands, it can contract back to its original size and shape when cooled down to its initial temperature. This reversible expansion and contraction phenomenon is observed in many materials, such as metals and certain solids.
Que. How is the expansion of substances utilized in everyday life?
Sol. The expansion of substances due to heating is utilized in various practical applications. For example, the bimetallic strips used in thermostats take advantage of the different expansion rates of two metals to control temperature. Expansion joints in bridges and railways allow for the expansion and contraction of materials due to temperature changes, preventing damage.
Que. Are there any exceptions to the rule that substances expand on heating?
Sol. There are a few exceptions to the general rule that substances expand on heating. One notable exception is water. Water contracts upon cooling from 0°C to 4°C, reaching its maximum density at 4°C. However, as it is further cooled below 4°C or heated above 4°C, it expands, following the typical behavior of most substances.
Que. Can the expansion of a substance lead to structural damage?
Sol. Yes, the expansion of a substance can potentially cause structural damage, especially when it is constrained or unable to freely expand. If the expansion is restricted, the internal stress can build up, leading to cracks, fractures, or warping. Proper design and consideration of expansion and contraction are crucial in many engineering and construction applications.
Que. How is the expansion of substances related to the concept of thermal expansion?
Sol. The expansion of substances is a manifestation of thermal expansion, which refers to the increase in size or volume of a substance due to an increase in temperature. Thermal expansion is a fundamental property of matter and is governed by the coefficient of thermal expansion, which quantifies the rate of expansion for a given substance.
Que. Are there any practical precautions to consider regarding the expansion of substances?
Sol. When working with substances that exhibit significant expansion upon heating, it is important to consider the potential effects of thermal expansion. This includes allowing for adequate expansion space in structures, using expansion joints, and considering the thermal expansion properties of materials when designing systems or devices.