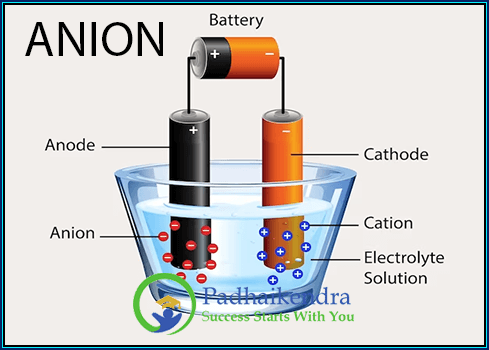
Anion is an ion that has a negative charge due to the gain of one or more electrons. Anions are formed when an atom or molecule gains one or more electrons to achieve a stable electronic configuration, usually the configuration of the nearest noble gas.
For example, when a chlorine atom gains an electron, it forms a chloride ion with a single negative charge (Cl-). Similarly, when an oxygen atom gains two electrons, it forms an oxide ion with a double negative charge (O2-).
Anions play important roles in many chemical reactions and in the properties of compounds. For example, ionic compounds are formed when cations (positively charged ions) and anions come together due to electrostatic attraction. Anions also contribute to the chemical reactivity and solubility of compounds.
Anions can be identified in chemical reactions using various analytical techniques, such as spectroscopy, chromatography, and electrochemistry.
Anion FAQs
An anion is a negatively charged ion that is formed when an atom or molecule gains one or more electrons. Anions are attracted to positively charged ions, called cations, and are an essential part of many chemical reactions and compounds.
Anions form through a process called ionization or electron gain. When an atom or molecule gains one or more electrons, it becomes negatively charged, resulting in the formation of an anion. This electron gain can occur through various chemical reactions, such as the transfer of electrons or the sharing of electron pairs.
An anion is a negatively charged ion, while a cation is a positively charged ion. Anions are formed when atoms or molecules gain electrons, while cations are formed when they lose electrons. These opposite charges allow anions and cations to attract each other and form ionic compounds.
Some common examples of anions include chloride (Cl^-), sulfate (SO4^2-), nitrate (NO3^-), carbonate (CO3^2-), and hydroxide (OH^-). These anions are frequently encountered in various chemical compounds, such as table salt (sodium chloride, NaCl) and sulfuric acid (H2SO4).
Anions are typically represented by writing the chemical symbol of the atom or atoms followed by the appropriate charge. The charge is indicated as a superscript after the chemical symbol. For example, the chloride ion is represented as Cl^-, and the nitrate ion is represented as NO3^-.
Anions generally have larger sizes compared to their parent atoms due to the addition of extra electrons. They are attracted to positively charged species and can participate in ionic bonding. Anions may also exhibit specific chemical reactivity and play important roles in chemical reactions and the formation of compounds.
Anions can exist independently in certain cases, such as when they are part of a stable compound or in an ionic solution. However, in isolation or free form, anions are often unstable due to their tendency to attract cations and achieve a balanced charge. In a neutral state, anions are commonly found in association with cations.
Anions significantly influence the properties of compounds. They contribute to the solubility, reactivity, and conductivity of substances. Anions can determine the pH level, acidity or alkalinity, and the behavior of compounds in various chemical reactions. The presence of specific anions can also impact the color, stability, and other physical and chemical characteristics of compounds.