The boiling point of a substance is defined as the temperature at which its vapor pressure equals the atmospheric pressure. The relationship between pressure and boiling point is well established, and it can be observed that increasing the pressure on a substance generally results in an increase in its boiling point. This relationship holds true for water as well.
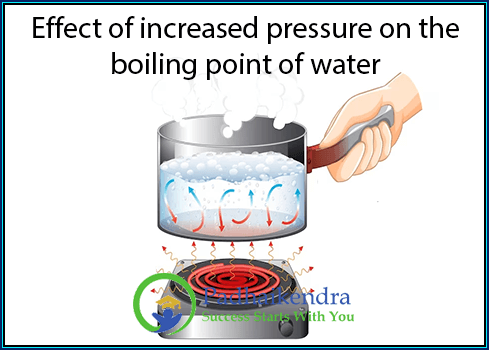
Effect of Increased Pressure on the Boiling Point of Water
When pressure is increased, the vapor pressure required for a liquid to transition into its gaseous state also needs to increase in order to overcome the higher external pressure. As a result, the temperature at which the vapor pressure of the liquid equals the increased pressure (atmospheric pressure plus the applied pressure) will be higher than the normal boiling point.
For example, at standard atmospheric pressure (1 atmosphere), the normal boiling point of water is 100 degrees Celsius (212 degrees Fahrenheit). However, if the pressure is increased, such as in a pressure cooker, the boiling point of water can rise above 100 degrees Celsius.
Working of Pressure Cooker
This is why pressure cookers are used in cooking. By increasing the pressure inside the cooker, the boiling point of water is elevated, allowing food to cook at higher temperatures. The higher temperature results in faster cooking times and improved texture and flavor.
Conversely, if the pressure is decreased, such as at high altitudes where atmospheric pressure is lower, the boiling point of water decreases. This is why it takes longer to cook food or boil water at high altitudes where the boiling point is lower than the standard 100 degrees Celsius.
Increasing the pressure on the water leads to an increase in its boiling point. The relationship between pressure and boiling point is directly proportional, with higher pressure requiring a higher temperature for the liquid to boil. This phenomenon has practical applications in various fields, including cooking, industrial processes, and scientific research.