Inertness of Noble Gases
Noble gases are a group of elements that are chemically inert, meaning that they do not easily react with other elements. This is because they have a full outer electron shell, which gives them a stable electronic configuration.
The noble gases, also known as inert gases, include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These elements are known for their extremely low reactivity and are often referred to as inert because they rarely react with other elements to form compounds.
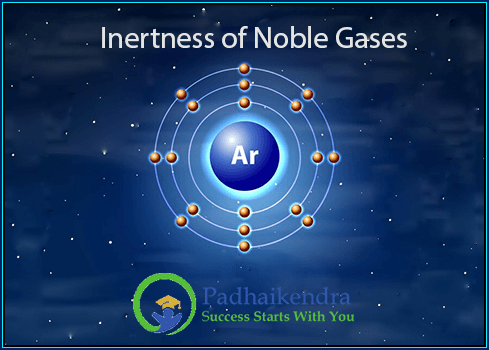
The inertness of noble gases can be explained by their electronic configuration. The outer electron shell of a noble gas atom is full i.e. noble gases have a full valence shell, which means that it has eight electrons. This is the maximum number of electrons that can fit in the outer shell.
When an atom has a full outer electron shell, it is very stable and does not need to react with other atoms to gain or lose electrons. This is why noble gases are inert.
The full valence shell of noble gases also explains why they are found in their elemental form in nature. Because they do not readily react with other elements to form compounds, they are often found in their pure, uncombined state in the atmosphere and in the Earth’s crust.
There are a few exceptions to the inertness of noble gases. They can be induced to form compounds under certain extreme conditions, such as high pressure or high temperature. These compounds, known as “noble gas compounds”, are highly unstable and typically decompose quickly.
Example- Xenon can form compounds with fluorine and oxygen. These compounds are called xenon fluorides and xenon oxides.
Radon is also a bit more reactive than the other noble gases. It can form compounds with oxygen and chlorine.
Overall, noble gases are very inert elements. They are important in many different applications, such as lighting, welding, and medical imaging.
Applications of Noble Gases
Noble gases have a variety of applications in different industries.
Lighting: Noble gases are used in fluorescent lamps and neon signs.
Welding: Argon is used as a shielding gas in welding to prevent the oxidation of the metal being welded.
Medical imaging: Radon is used in medical imaging, such as CT scans, to detect tumors and other abnormalities in the body.
Future of Noble Gas Research
Researchers are continuing to study the properties of noble gases and their potential applications. Some of the areas of active research include:
The development of new compounds with noble gases: Researchers are working to develop new compounds with noble gases, such as xenon fluorides and xenon oxides. These compounds could have potential applications in a variety of industries.
The use of noble gases in medical research: Researchers are studying the use of noble gases in medical research. For example, radon is being used to study the effects of radiation on the body.
The use of noble gases in space exploration: Noble gases could be used in space exploration to provide shielding from radiation and to create atmospheres on other planets.