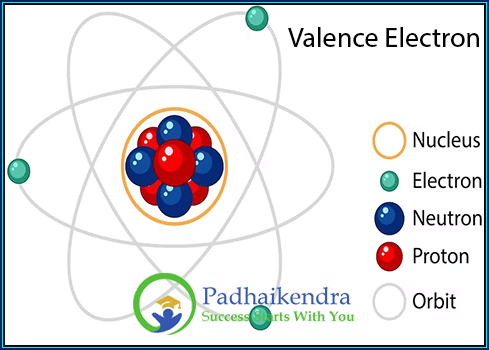
What are Valence electrons?
Valence electrons are the electrons located in the outermost energy level or shell of an atom. These electrons are involved in chemical bonding and determine the reactivity and chemical properties of an element.
The number of valence electrons an atom has corresponds to its position in the periodic table. In general, main group elements (Groups 1, 2, 13-18) have valence electrons equal to their group number. For example, elements in Group 1, such as hydrogen (H), lithium (Li), and sodium (Na), have one valence electron, while elements in Group 2, such as beryllium (Be) and magnesium (Mg), have two valence electrons.
For elements in Groups 13-18, the number of valence electrons can be determined by subtracting the group number from ten. For instance, carbon (C) in Group 14 has four valence electrons (10 – 6 = 4), while oxygen (O) in Group 16 has six valence electrons (10 – 8 = 6).
Valency
The valence electrons of an atom determine its valency or the number of bonds it can form with other atoms. The valency of an element is related to its position in the periodic table and is typically equal to the number of valence electrons it has.
For example, carbon has four valence electrons, which allows it to form up to four chemical bonds with other atoms, while oxygen has six valence electrons and can form up to two chemical bonds.
Representation of Valence Electrons
 The valence electrons are typically represented as dots around the symbol of the element, using the electron dot notation. In this notation, the symbol of the element represents the nucleus and all of the inner electrons, while the dots represent the valence electrons.
The valence electrons are typically represented as dots around the symbol of the element, using the electron dot notation. In this notation, the symbol of the element represents the nucleus and all of the inner electrons, while the dots represent the valence electrons.
Valence electrons play a critical role in chemical reactions. Elements tend to gain, lose, or share electrons to achieve a stable electron configuration, often following the octet rule. This rule states that atoms will gain, lose, or share electrons to attain a full outer shell of eight electrons (except for hydrogen and helium, which only require two electrons).
The behavior of Valence Electrons
The behavior of valence electrons determines an element’s ability to form bonds, its chemical reactivity, and the types of compounds it can form. Elements with a full complement of valence electrons (noble gases) tend to be chemically inert since they have little tendency to gain or lose electrons. In contrast, elements with few valence electrons are more likely to react with other elements to achieve a stable electron configuration.
Understanding the number and behavior of valence electrons is crucial in predicting and explaining the formation of chemical bonds, the reactivity of elements, and the properties of compounds