Introduction to Allotropy
In the vast expanse of the scientific world, terms and concepts often emerge that might sound intricate at first but are deeply rooted in everyday phenomena. One such term that holds notable importance in the world of chemistry and material science is “allotropy”.
What is Allotropy?
Allotropy refers to the phenomenon where a single chemical element can exist in two or more different structural forms, each distinct in its physical or chemical properties. These different forms are called allotropes. Imagine having the same set of building blocks, but arranging them in different patterns to create diverse structures. Similarly, atoms of an element come together in varied arrangements to form different allotropes.
For example, one of the most commonly cited illustrations of allotropy is that of carbon. The same carbon atoms are arranged in different ways to give us a diamond, where every atom is bonded to four other carbon atoms in a tetrahedral shape, and graphite, where each carbon atom is bonded to three others in flat, 2D planes. Though both are forms of carbon, their properties are worlds apart; diamond is a hard, transparent gemstone, while graphite is a black, opaque, and slippery material.
Significance in Chemistry and Material Science
Allotropy is not just a theoretical concept reserved for textbooks; it has profound implications in both chemistry and material science. Here’s why it’s significant:
Understanding Material Properties: As seen in the carbon example, the different allotropes of an element can have starkly different physical and chemical properties. Recognizing these differences is crucial for scientists and engineers when selecting or designing materials for specific applications. For instance, while diamond’s hardness makes it ideal for cutting tools, graphite’s ability to conduct electricity and its lubricating properties find applications in batteries and machinery.
Chemical Reactions: The reactivity of an element can change based on its allotropic form. For example, white phosphorus is much more reactive than red phosphorus, even though both are forms of the same element. Such knowledge is vital for industries that rely on chemical reactions, as using the wrong allotropic form can lead to unexpected or undesirable results.
Innovation in Material Science: Understanding allotropy can lead to groundbreaking innovations. For instance, researchers have been excited about graphene (another allotrope of carbon) due to its exceptional conductivity, strength, and flexibility. This could pave the way for advancements in electronics, energy storage, and even medical devices.
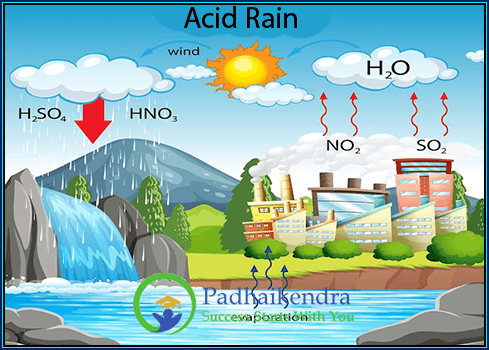
Environmental Implications: Certain allotropes play pivotal roles in the environment. Ozone, an allotrope of oxygen, forms a protective layer in our atmosphere that shields us from harmful ultraviolet radiation.
Allotropy is a captivating phenomenon that offers insights into the flexibility of elements to exist in multiple forms. This not only deepens our understanding of the elemental nature but also empowers scientists and industries to harness the right form of the element for specific applications, leading to technological advancements and a better understanding of our world.
Basics of Allotropy
The realm of material science is rich with phenomena that highlight the versatile nature of elements and compounds. At the heart of these phenomena lies allotropy. But before delving deeper into the essence of allotropy, it’s crucial to distinguish it from a concept that often gets mistaken for it – polymorphism.
Difference between Allotropy and Polymorphism
Both allotropy and polymorphism involve a substance existing in two or more different forms. The primary distinction lies in the type of substance:
Allotropy: Refers to the existence of different forms of the same element in the same physical state, due to different arrangements of atoms. For instance, diamond and graphite are both allotropes of carbon.
Polymorphism: Relates to the occurrence of different structural forms of the same compound. A classic example of polymorphism is calcium carbonate, which can exist as calcite, aragonite, and vaterite – each with its unique structure but the same chemical composition.
Allotropism Versus Polymorphism
While the terms are distinct, the underlying principle is the versatility of matter:
- Allotropism showcases the diversity an element can exhibit based solely on how its atoms are arranged. The fascinating part is that these different arrangements can lead to vastly different properties even though the building blocks (atoms) remain unchanged.
- Polymorphism is a testament to the fact that even when atoms come together to form a compound, they can do so in multiple ways. This ability to adopt varied structures adds layers of complexity to the behavior and characteristics of compounds.
Properties of Polymorphism
Polymorphism, by nature, influences the properties of compounds:
Physical Properties: Different polymorphs often have variations in hardness, melting point, and solubility. For example, two polymorphic forms of a drug might dissolve at different rates in the body, leading to varied therapeutic effects.
Stability: Some polymorphs are more stable than others under specific conditions. Over time, a less stable polymorph can convert to a more stable form.
Optical Properties: Polymorphs can exhibit different optical properties, influencing the transparency, refractive index, or even color of a substance.
Conditions Leading to Allotropy
The formation of different allotropes typically hinges on certain conditions:
Temperature: Variations in temperature can lead to a shift from one allotropic form to another. For example, rhombic sulfur transforms into monoclinic sulfur when the temperature rises above 95.3°C.
Pressure: High pressures can induce the formation of certain allotropes. Diamonds, for instance, form under immense pressures deep within the Earth.
Other Environmental Factors: Factors like radiation or the presence of certain chemicals can also influence allotropic transformations.
Properties of Allotropes
Differing atomic arrangements in allotropes lead to varied properties:
Physical Properties: Hardness, color, density, and electrical conductivity can vary significantly among different allotropes. While graphite is a good conductor of electricity, diamond is an insulator.
Chemical Reactivity: Different allotropes might react differently with other substances. White phosphorus is highly reactive, whereas red phosphorus is much less so.
Optical Properties: Some allotropes can be transparent, like a diamond, while others, such as graphite, are opaque.
The study of allotropy provides profound insights into the structural versatility of elements, underscoring the profound influence of atomic arrangement on material properties. Polymorphism, a parallel concept for compounds, serves as a reminder of the intricate ways in which atoms can combine, even when forming the same substance.
Examples and Details of Allotropes
Allotropy is a captivating testament to nature’s adaptability, proving that even single elements can have multifaceted personalities. The varied forms, or allotropes, of an element, can have drastically different properties due to unique atomic arrangements.
Carbon: A Versatile Element
Diamond: These sparkling gemstones are more than just eye candy. Each carbon atom in a diamond is tetrahedrally bonded to four other carbons. This strong covalent bonding gives a diamond its remarkable hardness.
Graphite: Used in pencils and as an industrial lubricant, graphite is composed of layers of carbon atoms arranged in hexagons. These layers can easily slide over each other, making graphite slippery. Unlike diamond, graphite is a good conductor of electricity.
Fullerenes: Resembling soccer balls, fullerenes are molecules made entirely of carbon. The most famous fullerene is C₆₀, resembling a hollow sphere.
Graphene: A single layer of carbon atoms arranged in a hexagonal lattice. It’s renowned for its strength and conductivity, leading to significant interest in electronics and other technological applications.
Amorphous Carbon: This is a catch-all term for carbon allotropes that don’t fit into the neat categories of diamond, graphite, etc. Examples include coal, charcoal, and soot.
Properties, Occurrence, and Uses (Specific to Carbon): Carbon is life’s foundational element. Its allotropes, as mentioned, find uses in jewelry (diamond), writing (graphite in pencils), nanotechnology (graphene), and energy (fullerenes in solar cells).
Allotropes of Oxygen
O₂ (Dioxygen): The breathable form of oxygen and a crucial component of our atmosphere.
O₃ (Ozone): A triatomic molecule that forms a protective layer in the Earth’s stratosphere, shielding us from the Sun’s harmful ultraviolet rays.
Allotropes of Sulfur
Sulfur: The Yellow Allotrope of the 16th Group
Rhombic Sulfur: The most stable form of sulfur at room temperature, it has an octahedral crystal structure.
Monoclinic Sulfur: Exists in needle-like crystals and becomes stable at temperatures above 95.3°C, reverting to rhombic sulfur upon cooling.
Allotropes of Phosphorus
Phosphorus: The Fiery Element
White Phosphorus: Consisting of P₄ tetrahedra, it’s highly reactive, even spontaneously igniting in air.
Red Phosphorus: An amorphous network structure makes red phosphorus more stable and less reactive than its white counterpart.
Black Phosphorus: Has a layered structure, similar to graphite. It’s the least reactive form of phosphorus and has semiconducting properties.
Other Noteworthy Allotropes
Tin: With two allotropes, gray and white tin, it’s noteworthy for the “tin pest” phenomenon. Below 13.2°C, white tin slowly turns into the gray form, which can compromise tin objects in cold climates.
Iron: Has several allotropes that differ in their crystal structures, playing a significant role in steel-making processes.
An Element that Doesn’t Exhibit Allotropy:
Gold is a notable example. It exists in only one form in nature under ambient conditions, making it a monomorphic element.
The realm of allotropy is vast, with each element showcasing its unique dance of atomic arrangements. These different arrangements underscore the richness of the elemental world, providing insights into the myriad of ways atoms can come together to sculpt the world around us.
Impact of Allotropy on Material Properties and Uses
Allotropy, the phenomenon wherein an element can exhibit multiple structural forms, has significant consequences for the properties of materials. These alterations can radically influence an element’s practical applications in industries ranging from electronics to jewelry.
Influences on Material Properties
Strength and Hardness: The atomic arrangement within allotropes can lead to variance in strength and hardness. Diamond, an allotrope of carbon, stands as a testament to this. Its tetrahedral atomic structure provides it with remarkable hardness, making it the hardest known natural material.
Electrical Conductivity: The electron arrangement in different allotropes can alter their conductive properties. For instance, graphite’s planar structure allows electrons to move freely, making it a good conductor. In contrast, a diamond lacks this free electron movement, rendering it an insulator.
Optical Properties: Allotropy can influence a material’s transparency, refractivity, and even color. Diamond, being transparent, allows light to pass and refract, giving its characteristic sparkle. In contrast, graphite is opaque.
Reactivity: Different allotropes can have varying levels of reactivity. White phosphorus is notorious for its reactivity, even igniting spontaneously in air, while red phosphorus is comparatively stable.
Production and Uses of Allotropes
Understanding and harnessing allotropy can pave the way for producing specific allotropes intentionally. For example:
Diamonds, while naturally occurring, can now be synthetically produced under high-pressure, high-temperature conditions.
Fullerenes and graphene, newer carbon allotropes, are produced using advanced techniques like chemical vapor deposition and epitaxy.
Buckminsterfullerene: Buckminsterfullerene is a spherical molecule with a cage-like structure. It is a very strong material, and it is being investigated for a variety of applications, including in electronics and medicine.
Practical Applications
Electronics: The discovery of graphene, an allotrope of carbon, stirred excitement in the electronics industry. With its remarkable electrical conductivity and thinness, graphene is considered a potential replacement for silicon in future electronic devices.
Jewelry: Diamonds have long been cherished for their brilliance and have become symbols of luxury and commitment in jewelry.
Industrial Applications: Graphite’s lubricating properties make it valuable in machinery. Its conductivity also finds use in batteries. Meanwhile, the hardness of diamonds makes them essential for cutting tools.
Environmental Implications: Ozone, an allotrope of oxygen, forms a protective layer in the Earth’s stratosphere. This ozone layer plays a critical role in absorbing the majority of the Sun’s harmful ultraviolet rays, safeguarding life on Earth.
Allotropy does more than just showcase the flexible personalities of elements; it drives advancements across sectors. From the dazzling allure of diamonds to the groundbreaking potential of graphene in electronics, the influence of allotropy reverberates through science, industry, and our daily lives.
Methods to Induce or Change Allotropy
The ability of an element to exhibit different allotropic forms offers myriad possibilities in science and technology. However, transitioning from one allotropic form to another requires specific conditions or triggers.
Pressure and Temperature Conditions
Diamond and Graphite: The creation of synthetic diamonds illustrates the influence of pressure and temperature. Natural diamonds form deep within the Earth’s mantle under extreme conditions. Replicating these conditions, manufacturers subject graphite to high pressures and temperatures, converting it into a diamond.
Sulfur Allotropes: As discussed earlier, rhombic sulfur transitions to monoclinic sulfur at temperatures above 95.3°C. Upon cooling, it reverts to its rhombic form, showcasing the temperature’s pivotal role.
Iron Allotropes: Iron exhibits several allotropes that arise at different temperatures, influencing the steel-making process. For instance, at room temperature, iron is in the α-ferrite form but transitions to γ-iron (austenite) at around 912°C.
Chemical Methods
Certain chemical reactions or treatments can induce a shift from one allotropic form to another:
Amorphous to Crystalline: Some elements can be converted from their amorphous (non-crystalline) form to a crystalline form via specific chemical treatments. For instance, amorphous silicon, when heated with a trace of metal, can convert into its crystalline form.
Reduction or Oxidation: The oxidation state of an element can influence its allotropic form. For example, certain allotropes might form or be stabilized by either adding or removing electrons through chemical reactions.
Use of Catalysts
Catalysts are substances that can speed up a reaction without undergoing any permanent change themselves. They can play a role in the formation of certain allotropes:
Graphene Production: The production of high-quality graphene often employs copper as a catalyst. In chemical vapor deposition, a gaseous carbon source decomposes onto a copper substrate, forming a single layer of graphene.
Fullerenes Production: Certain metal surfaces can catalyze the formation of fullerenes from carbon sources, guiding the carbon atoms into the desired spherical arrangements.
Manipulating allotropy is like wielding nature’s toolkit, allowing scientists to tailor materials to specific needs. Whether it’s through pressure, temperature, chemical reactions, or catalysts, understanding and controlling these changes offers vast potential in material science and myriad other fields.
Abundance, Isotopes, and Compounds
The diversity and versatility of elements and compounds extend beyond just their molecular and structural forms. A more profound understanding emerges when we look into their abundance on Earth, the existence of isotopes, and how even compounds can exhibit behaviors akin to allotropy.
Abundance and Isotopes: Exploring Natural Occurrence
Abundance: Abundance refers to how frequently an element or compound occurs in nature. For instance, oxygen is the most abundant element in the Earth’s crust, making up almost 47% of its weight. Similarly, silicon is second, constituting 28% of the crust.
Isotopes: Atoms of the same element having the same number of protons but different numbers of neutrons are termed isotopes. This difference in neutron number gives each isotope unique properties:
Carbon Isotopes: Carbon primarily exists as carbon-12 (⁶C₁₂) with 6 protons and 6 neutrons. However, carbon-14 (⁶C₁₄) is a rare isotope with 8 neutrons, which is radioactive and used in radiocarbon dating.
Hydrogen Isotopes: Hydrogen has three isotopes – protium (¹H), deuterium (²H), and tritium (³H). Deuterium, having one proton and one neutron, is used in heavy water, while tritium, radioactive with two neutrons, finds applications in nuclear reactors.
Isotope abundance can provide insights into elemental origins, ages, and processes. For example, the ratio of stable isotopes of oxygen (¹⁶O and ¹⁸O) in ice cores offers clues about past climates.
Allotropic Compounds: How Compounds Can Exhibit Allotropy
While “allotropy” typically pertains to elements, the concept can be extended to compounds, though it’s more accurately termed “polymorphism”. Compounds can exist in multiple structural forms due to different arrangements of their molecules or ions.
Calcium Carbonate: This compound exists in three predominant forms – calcite, aragonite, and vaterite. While chemically identical, each form has a unique crystal structure that endows it with distinct properties. For example, calcite and aragonite are two common minerals in marine organisms, but they differ in hardness and crystal symmetry.
Silica (SiO₂): Silica, a compound vital for many technological applications, exhibits polymorphism. Quartz, tridymite, and cristobalite are some polymorphs of silica, each with its crystal arrangement.
Organic Compounds: Many organic compounds, especially pharmaceuticals, exhibit polymorphism. A drug’s efficacy, solubility, and stability can depend on its specific crystalline form. This makes understanding and controlling polymorphism crucial in drug development.
The study of abundance reveals the natural distribution of elements, isotopes shed light on the nuanced variations within elements, and the exploration of allotropic compounds (or polymorphs) offers insights into the diverse ways compounds can arrange themselves.
Future Prospects and Research on Allotropy
Allotropy, as a foundational concept in material science, has been at the forefront of numerous groundbreaking discoveries over the years. As science and technology evolve, the horizons of allotropy research expand, presenting promising prospects for the future.
Potential New Allotropes
With advancements in experimental techniques and computational modeling, scientists are on the hunt for previously undiscovered allotropes.
High-Pressure Research: Extreme pressures can give rise to unique atomic arrangements. Using diamond anvil cells, researchers are exploring the existence of novel allotropes under conditions not naturally found on Earth’s surface.
Advanced Computational Models: Before conducting lab experiments, scientists are now able to use computer simulations to predict the existence and properties of potential new allotropes. This predictive capability can streamline the discovery process and pinpoint promising candidates.
Interdisciplinary Collaboration: Bringing together experts from fields like physics, chemistry, and engineering can foster innovative approaches to uncovering new allotropes. This holistic approach enriches the pool of knowledge and techniques available for exploration.
Applications in Nanotechnology and Quantum Computing
Allotropy’s potential in the domains of nanotechnology and quantum computing is profound:
Nanotechnology:
Graphene: This one-atom-thick layer of carbon has caught the world’s attention due to its remarkable properties. Its strength, combined with its flexibility and conductivity, makes it a promising candidate for creating super-strong nano-materials and next-generation electronic devices.
Fullerenes: These spherical carbon structures are being explored as potential drug delivery vehicles, especially in targeted cancer therapies. Their hollow nature allows for the encapsulation of drug molecules.
Quantum Computing:
Diamond Allotropes with Nitrogen-Vacancy (NV) Centers: Diamonds with specific impurities (like a missing carbon atom adjacent to a nitrogen atom) can trap electrons in a unique quantum state. These NV centers are being studied as qubits—the fundamental units in quantum computing. The stability and coherence of NV centers in diamonds could pave the way for scalable and more efficient quantum computers.
Topological Insulators: Some new allotropes are being researched for their potential as topological insulators—materials that are insulators inside but can conduct on their surface. They have potential applications in quantum computing due to their unique electronic properties.
The realm of allotropy, far from being a settled science, is brimming with potential. As our technological capabilities grow, our understanding of allotropy will expand, potentially unlocking materials and applications previously considered the stuff of science fiction. The future prospects in allotropy research are not just about new discoveries but about refining our world and reshaping the future of technology.
Allotropy related FAQs
- Carbon: diamond, graphite, and buckminsterfullerene
- Oxygen: diatomic oxygen (O2) and ozone (O3)
- Sulfur: rhombic sulfur, monoclinic sulfur, and amorphous sulfur
- Phosphorus: white phosphorus, red phosphorus, and black phosphorus
- Tin: white tin, gray tin, and beta tin
- Diamond is used in jewelry, cutting tools, and drill bits.
- Graphite is used in pencils and lubricants. It is also a good conductor of electricity, and it is used in batteries and other electronic devices.
- Buckminsterfullerene is being investigated for a variety of applications, including in electronics and medicine.